CryoCord’s stem cell research fuels hope for a healthier Malaysia.
Stem cell therapy, often hailed as regenerative medicine’s next frontier, holds immense promise in revolutionising the treatment of a wide range of diseases and injuries. The unique ability of stem cells to self-renew and differentiate into various cell types makes them a powerful tool for repairing damaged tissues and organs. From treating blood cancers and autoimmune diseases to potentially reversing the effects of neurodegenerative disorders, the potential applications of stem cell therapy are vast and continue to expand.
However, unlocking the full potential of stem cell therapy requires continuous research and development. Through dedicated R&D efforts, exemplified by institutions like CryoCord, scientists can delve deeper into the complexities of stem cell biology, refine existing techniques, and discover novel therapeutic approaches. R&D plays a crucial role in improving the safety and efficacy of stem cell treatments, ensuring that patients receive the most effective and reliable care possible. Moreover, R&D is essential for expanding the scope of stem cell therapy, enabling the development of treatments for currently incurable diseases, and improving the quality of life for numerous individuals.
Groundbreaking Research
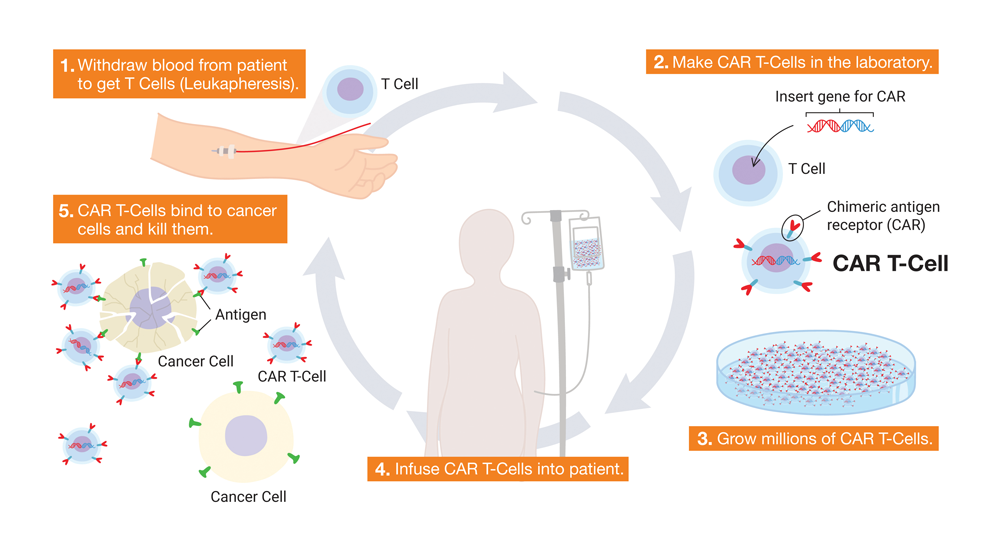
Besides stem cell therapy, CryoCord is also engaged in extensive research and development across various promising areas within gene therapy and immunotherapy. A key focus area is CAR (chimeric antigen receptor) T-cell therapy, a novel approach to cancer treatment. This therapy harnesses the patient’s own immune system to target and eliminate cancer cells.
The process involves extracting the patient’s T-cells (a type of white blood cell) and genetically modifying them to recognise specific antigens present on cancer cells. These modified T-cells, known as CAR T-cells, are then reintroduced into the patient’s body, where they can identify and destroy cancer cells with high precision.
CryoCord’s commitment to CAR T-cell therapy research stems from its potential to provide a more targeted treatment option compared to conventional chemotherapy or radiation. This is particularly crucial in the context of childhood cancers, where the long-term impact of harsh side effects from standard treatments can significantly affect a patient’s quality of life.
In 2012, Emily Whitehead, a seven-year-old with relapsed acute lymphoblastic leukaemia (ALL), became the first paediatric patient to receive CAR T-cell therapy. After two relapses and with no remaining conventional treatment options, Emily was enrolled in a CAR T-cell therapy clinical trial at Children’s Hospital of Philadelphia (CHOP). This groundbreaking therapy, developed at CHOP and the University of Pennsylvania. The treatment proved successful, leading to Emily’s complete remission and paving the way for FDA approval of CAR T-cell therapy for paediatric and young adult patients with relapsed/refractory ALL and diffuse large B-cell lymphoma (DLBCL). Twelve years after treatment, she remains cancer-free.
Leading the Way in CAR T-cell Therapy Research
CryoCord is dedicated to advancing gene therapy research, demonstrated by its active involvement in the development of CAR T-cell therapy. Plutonet, a subsidiary of the CryoCord Group of Companies, has partnered with HUKM (Hospital Universiti Kebangsaan Malaysia) to initiate two clinical trials focusing on Non-Hodgkin Lymphoma (NHL) and Acute Lymphoblastic Leukemia (ALL). This research holds significant promise for enhancing treatment outcomes and potentially achieving cures for various cancers.
Currently, CAR T-cell therapy has has been approved by the FDA for the following indications:
- B-Cell Acute Lymphoblastic Leukaemia
- B-Cell Non-Hodgkin Lymphoma
- Multiple Myeloma
Recent research indicates that CAR T-cell therapy has shown promising potential in treating lupus.
In addition to CAR T-cell therapy, CryoCord is pursuing other notable research and development projects that investigate the therapeutic applications of stem cells for a wide array of diseases and conditions. These endeavours further solidify CryoCord’s position as a leader in the field of regenerative medicine.
This commitment to research, coupled with a state-of-the-art blood cord storage facility, positions CryoCord as a leader in the field. By pushing the boundaries of scientific knowledge and ensuring the optimal preservation of stem cells, CryoCord is fostering hope for new and improved treatments, benefiting both individual patients and the advancement of medical science as a whole.
CryoCord is the BabyTalk MamaPapa Readers’ Choice Awards 2024 Diamond Award Winner for Cord Blood Storage.